The following is an excerpt from a news item on the U.S. Food and Drug Administration website.
by Susan Mayne, Ph.D., Center for Food Safety and Applied Nutrition Director
Consumers have an important role to play by choosing foods that can improve health, but the FDA is going further by encouraging industry innovation to create healthier products and helping consumers identify those products. Such innovation can also lead to an overall healthier food supply where healthier options are the norm. In just a year, the agency has made significant progress towards meeting the nutrition goals outlined in the Nutrition Innovation Strategy.
Putting Calories on the Menu
Providing calorie information on menus and menu boards, and other nutrient information on request, is a major initiative to make sure consumers have access to information to help them make healthy food choices while eating away from home. Although not every establishment is required to display caloric information, increasingly more and more restaurants and supermarkets are providing this information to consumers to help them make informed decisions about what they eat or buy. Only chain restaurants and other similar retail locations, such as grocery and convenience stores, that are part of a chain of 20 locations or more are required to comply with menu labeling requirements for standard menu items. In addition to listing calories where food choices are made, chains are also required to provide additional written nutrient information, such as disclosures for saturated fat, fiber and sodium, on request.
The FDA recently hit the year mark for implementation of menu labeling and over the past year the agency has done extensive outreach to establishments that are subject to menu labeling, including providing them with resources to help them comply with the requirements. It will take continued and consistent education to help restaurants and other retail locations comply fully with the new requirements, but the FDA is committed to continuing a cooperative approach to help industry achieve compliance. The agency also recognizes the importance of educating consumers by creating resources like Calories on the Menu — Information for Consumers.
Modernizing the Nutrition Facts Label
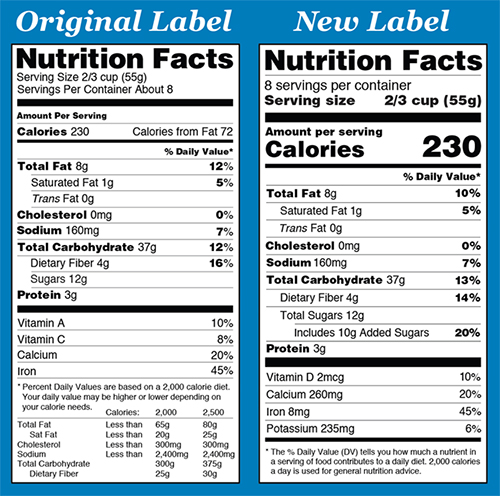
Similarly, there has been much progress implementing the new Nutrition Facts label on food packages. Even though the first compliance date isn’t until January 1, 2020, consumers may already see new labeling on products in supermarkets featuring caloric information in larger and bolder type, to draw attention to the information. There are also requirements for listing added sugars, and vitamin D and potassium are also newly required on the label. There are also other updates to the labeling that may not be as visible. For example, fiber added as an ingredient cannot count towards “Dietary Fiber” on the Nutrition Facts label unless science demonstrates it has a beneficial impact on health. Also, some nutrients have updated Daily Values that are reflected in the % Daily Value on the label.
The FDA is helping companies comply with the new labeling by providing several new guidance documents on issues related to serving sizes, format of the label, and other technical issues. The FDA intends to issue a final guidance on declaring added sugars on single-ingredient packages of sugars, syrups and cranberry products soon.
Labeling for Transparency and Clarity
The Nutrition Innovation Strategy addresses the need to modernize our approach to claims consumers see on food packaging. Public health recommendations for what is considered healthy when it comes to diet have evolved. Moreover, healthy dietary patterns now focus on food groups, the type of fat rather than the total amount of fat consumed, and now address excess added sugars in the diet. In light of these changes, and following extensive public input, FDA is working on a proposed rule to redefine the term “Healthy” when used as a nutrient content claim on a food package to update the criteria for this claim, which FDA intends to publish soon. More work on claims will follow.
FDA is also modernizing standards of identity as part of our nutrition strategy. Standards of identity are akin to recipes that indicate, among other things, what ingredients must be in a food. They have been helpful in making sure consumers are getting what they pay for and not less expensive alternatives, for example. But FDA wants to make sure these standards do not hinder the development of healthy products. The naming of plant-based beverages such as those that are soy-based using the term “milk,” is also an area being studied. The FDA issued a request for information to solicit feedback from our stakeholders on September 27, 2018, and we are reviewing more than 13,000 comments before moving forward to clarify such labeling.
